What is the PBS?
The Pharmaceutical Benefits Scheme, known as the PBS, is the Australian Government’s program to make medicines affordable. If a medicine is listed on the PBS, it will be sold at a really reduced price or available free under certain conditions.
Medicines on the PBS are subsidised so that patients pay a cheaper price for them. You may not know that you are using a medicine via the PBS. However, without the PBS many medications would be out of reach to patients who need them.
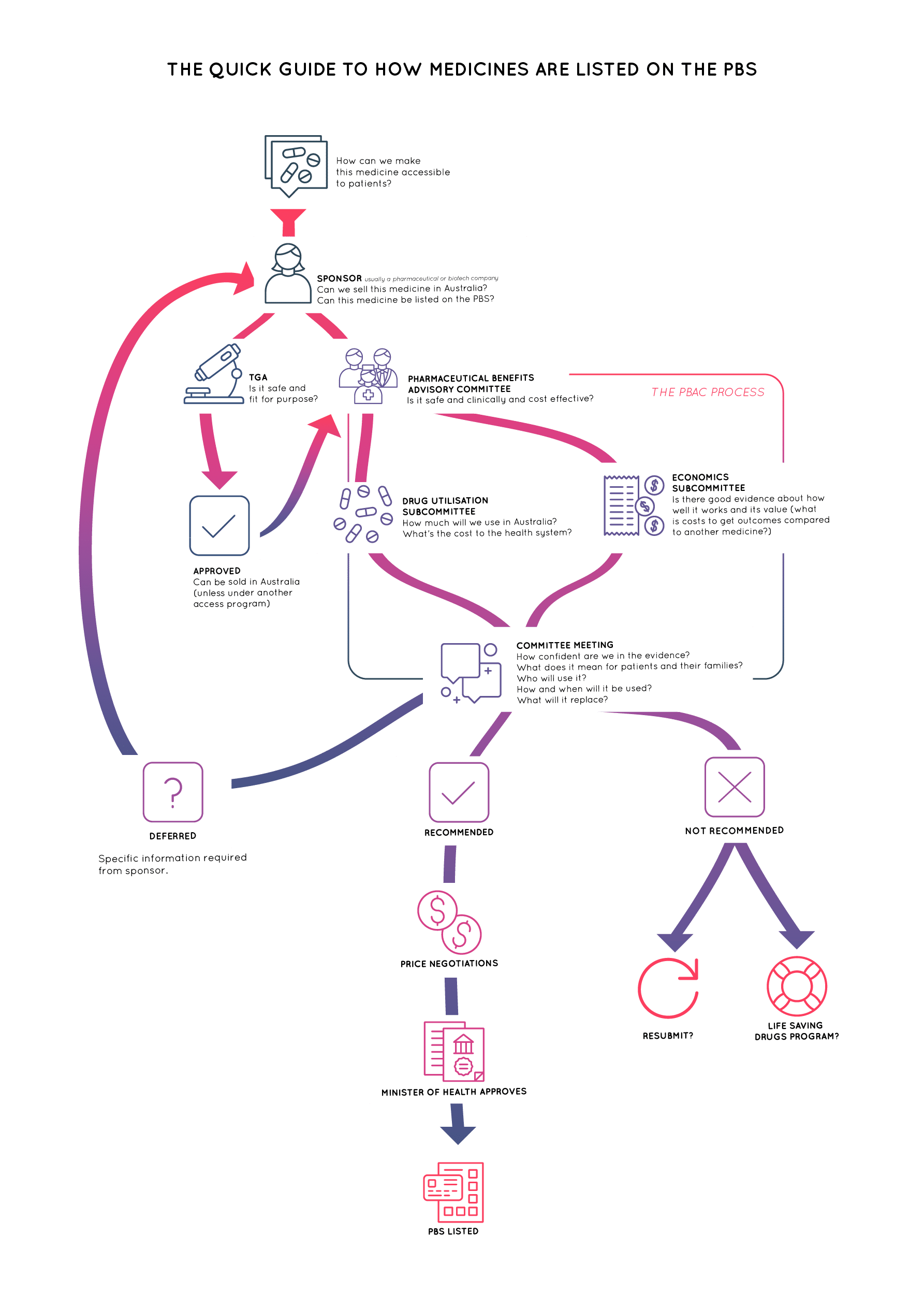
Because the amount of taxpayer funding available for medicines is limited, to be listed on the PBS, a medicine must be assessed as being good value for money. This assessment, known as a health technology assessment, is done by the Pharmaceutical Benefits Advisory Committee (PBAC).
Medicines on the PBS are subsidised so that patients pay a cheaper price for them. You may not know that you are using a medicine via the PBS. However, without the PBS many medications would be out of reach to patients who need them.
Because the amount of taxpayer funding available for medicines is limited, to be listed on the PBS, a medicine must be assessed as being good value for money. This assessment, known as a health technology assessment, is done by the Pharmaceutical Benefits Advisory Committee (PBAC).
The PBS and TGA both have useful videos and information on their websites if you’d like to find out more.
How does PBAC work?
The Pharmaceutical Benefits Advisory Committee is made up of doctors, health professionals, health economists and two consumer representatives; Jo Watson and Bel Harper. Jo is also Deputy Chair, with Professor Andrew Wilson as the Chair.
% of prescribed medicines subsidised by PBS
average days to reimbursement in 2018
weeks for PBAC assessment
Is this medicine safe, clinically effective, and cost-effective compared to an existing treatment?
ASSESSING THE VALUE OF MEDICINES
Before PBAC can advise the Minister to list a medicine on the PBS for reimbursement, the medicine must be approved by the Therapeutic Goods Association (TGA) for sale in Australia. Additionally, a sponsor (usually a pharmaceutical company) must make a submission to PBAC stating what the new medicine is, which patient population it is for and what the evidence is for its clinical and cost-effectiveness.
The evidence will also be data and models which are used to work out if the medicine is better value for money than the existing treatment (called the comparator). It might show which treatment provides more outcomes for the cost of treatment or which treatment costs the least for a given outcome.
Check out our Resource Library for more information on evidence and health technology assessments.

Find out more in the resource library.
What role does the Minister for Health play in listing new medicines?
You can have a voice.
– Albert Einstein
Despite all the evidence and experts involved in PBAC, there are still gaps and uncertainties in the information it must base its advice on.
This may be because something has not been measured in the evidence, or it is unknown how patients would value particular outcomes or trade-offs. Patients’ and caregivers’ lived experiences, preferences and needs can be invaluable when making sense of evidence.
For example, PBAC can see evidence about what happened to people on the clinical trials, but there may be important differences between you and the people on the trial, such as how and when they got the medicine or the stage or subgroup of their disease. And PBAC can see how the medicine compares with another medicine, but is there a more suitable comparator?
three ways of being involved
Consumer Comments
- Anyone can participate
- Send an online form (Written letter okay too)
Consumer hearings
- Participation by invitation only, mainly patient group representatives and PBAC representatives
- About six PBAC members meet with representatives to help PBAC understand some key issues and representatives to better understand information/evidence needed
Stakeholder meeting
- Participation by invitation only, mainly patient group representatives and sponsors
- Representatives from PBAC, sponsor and patient groups meet to try to work out issues when assessment is difficult, e.g. the medicine is the only potential option for a serious, disabling or life-threatening condition.
pbac assessment timeline
Sponsor makes submission
medicines being assessed published in agenda on pbac website
8 weeks later, all input including consumer comments, must be in
4 weeks later, pbac meets
6 weeks later, pbac outcome published
17 weeks from start to finish
pbac yearly timetable overview
Agenda published
- 26 July 2023
- 22 November 2023
- 3 April 2024
Deadline for Consumer Comments
- 20 September 2023
- 31 January 2024
- 29 May 2024
Meeting
- 1-3 November 2023
- 13-15 March 2024
- 10-12 July 2024
What happens to comments
When you submit a Consumer Comment, it is logged and blinded so the identity of the person who made the comment is not known by the PBAC Committee. It is then read by a Consumer Representative who looks for insights among the comments and groups them in themes. The Consumer Representative may have 1000s of comments to read in the four weeks before PBAC meets.
The Consumer Representative then summarises the insights before the meeting so that all committee members have access to these summaries alongside the evidence reports.
At the moment, there is no formal process for providing feedback on how useful the comments were or how they were taken account of when PBAC formed its advice. But, you can contact the Consumer Evidence and Engagement Unit or speak to a PBAC consumer representative once the decision has been made public.
How you can make a difference.
PBAC seeks comments from patients and the community to help them better interpret the evidence and to address gaps and uncertainties in the evidence. As the process already involves expert clinicians and researchers, Consumer Comments are a chance for PBAC to learn from the expertise gained from living with a condition. This is why patients and health consumers are sometimes called "lived experience experts".
So, what can you tell PBAC that other experts don’t know? You can inform them about:
Your (or your members’) unmet needs on the current treatment.
What outcomes matter most.
Differences in how healthcare/treatment is provided and what that might mean for patients.
Benefits of treatment (if you have tried it) or desired benefits (if you have not tried it).
Consequences of symptoms, side effects and benefits.
Consequences of rules for starting/stopping treatments.
Your usual treatment and if the comparator in the assessment is appropriate.

about pvi
Learn More
Don’t cut and paste
Tell your story
Don’t give general information
Be specific
Don’t give incidence
Explain what impact it had on you if it is a rare disease or condition
Don’t say ‘difficulty managing side effects’
Explain what the side effects are/were and what that meant for daily life
Don’t explain the condition
Explain the reality of living with the condition
Don’t explain the treatment
Explain the reality of having (or not having) treatment
Don’t submit clinical data
Submit what happens in the real world
Don’t submit published literature and stats
Consider collecting and contributing to studies that answer the questions you think are most important
Find out more: Tip Sheet for submitting Consumer Comment to the PBAC.